Caustic Layering
– the forgotten hazard
Ineffective
dissolution of solid caustic soda presents a risk of eruption, reports John Cox
 THE PHENOMENON of "Caustic
Layering", first identified in the 19th. Century, is rarely encountered
today. Suppliers of caustic soda have produced excellent technical literature
with clear warnings of the danger of creating weak and strong layers during
dissolution:
THE PHENOMENON of "Caustic
Layering", first identified in the 19th. Century, is rarely encountered
today. Suppliers of caustic soda have produced excellent technical literature
with clear warnings of the danger of creating weak and strong layers during
dissolution:
"It is important not to add the
pearls too quickly or they will tend to fall to the bottom and form a large agglomerate that will be slow to dissolve. Agitation
is also important to prevent the formation of a concentrated layer
of solution which would suddenly mix with a layer of weaker solution and cause
violent boiling".1
The boiling
risk may be evaluated using the enthalpy-composition diagram2 commonly
used to calculate the heat evolved when diluting a caustic soda solution. This
is illustrated on Fig.23 by the dotted line joining B1 (water at
65ºC) to B2 (a 55% solution at 77ºC). The line traces all the intermediate
mixtures (Point A, for example, with a composition of 27½% NaOH,
corresponds to a mixture of equal volumes of water and the 55% NaOH solution).
This procedure not only enables the temperature of a
mixture to be determined (for example, 112ºC for Point A) but also, if a
'Mixing line' cuts the 'Boiling Point curve', to indicate whether boiling will
occur during mixing. This is important because, although a simple 'splashback' is an ever-present hazard, a major eruption is
only possible with boiling.
The enthalpy-composition relationship also controls the
behaviour of strong and weak layers during mixing. In this instance however,
because both layers are already in the vessel, the mixing is more rapid. This
is why, for an equivalent energy release, the force of an eruption from
the mixing of layers is greater than from dilution or dissolution (which,
normally, takes place over a period of many seconds).
Although
Suppliers are fully aware of this hazard, it remains a mystery to some
Customers. As an illustration, a specific incident at a Resins Plant, and the
subsequent investigation and High Court litigation, is described in detail. The Company's RIDDOR report (8th.
July 1987) made their position clear from Day 1.
"An operator tending a resin
reactor (being caustic cleaned) reported to his shift manager having been
splashed with caustic soda solution. After preliminary treatment, drenching and
irrigation, he has been taken to hospital where concern has been expressed over
his eyes. No witness saw the accident but he has probably charged a bag of
caustic soda into the reactor containing hot caustic solution and been splashed
by the reactor".
This initial finding was endorsed by a formal
investigation which relied on an interpretation of the temperature record
(Chart 1). This showed a 3½ºC rise at 06.30am coinciding with whatever caused the eruption and subsequent
injuries to the process worker. As an extra 25kgs. of caustic soda would have caused a 2.7ºC rise, it was
concluded that a 25kg. bag must have been added.
This was already the consensus when the process worker
was interviewed in hospital some weeks later. No-one believed his claim to have
opened the inspection cover for a "quick
look inside" and that, after a "split-second",
the vessel erupted spontaneously. When he sought compensation, his Solicitors
were informed:
"The
accident occurred because contrary to (his) supervisors
instructions, he added solid caustic soda to a hot caustic cleaning solution in
a reactor. This caused an eruption through the manhole which resulted in injury
to your client. My client provided protective goggles and equipment for your
client. Your client was the author of his own misfortune and I am therefore not
able to advise my client to compensate (him)”.
Despite this rejection, the process worker persisted
with his story. He said that he had had no problem releasing and lifting the
strong-arm but some difficulty with a "sticky
seal" and that, when he lifted "the
cover slightly" (for a quick look inside),  "the
lid shot back" and
fluid splashed with such force as to "penetrate
his protective clothing and by-pass his goggles".
"the
lid shot back" and
fluid splashed with such force as to "penetrate
his protective clothing and by-pass his goggles".
The only hard evidence to substantiate this account was
his injuries - marked red on the drawings. These were clearly more credible for
the posture he described than that required if he had been discharging a bag of
caustic soda - notably:
· injuries to the
left inside arm: none to the right arm
· injuries to the
right knuckles: none to the left hand
· ingress of
caustic into the goggles:
more on left side
· 
 greater
facial injury on the left side and scalp
greater
facial injury on the left side and scalp
· no injuries to the torso or legs.
Although
this should have led the Company to consider whether the eruption could have
resulted from the disturbance of a layered solution, they did not do so. This
is astonishing since even a cursory scrutiny of the temperature record (Chart
1) reveals abnormal pre-accident operations by the previous night-shift.
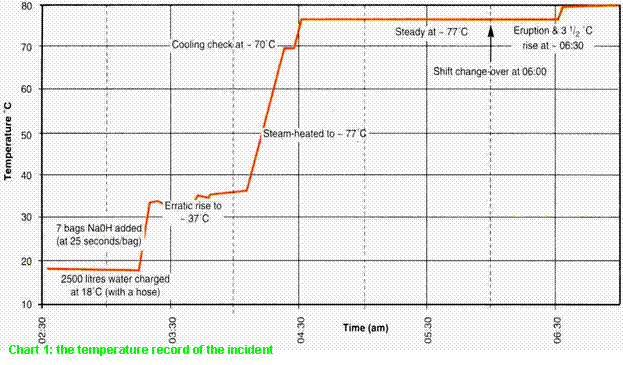
The basic procedures were supposed to
be:
· firstly, to charge 2500 litres of cool water (18ºC that day)
· then, to set the temperature controller to not exceed
~37ºC - and, about the same time, to start the agitator
· then to charge 8 25kg. bags of caustic soda (7 that day)
· then to steam-heat to ~80ºC (77ºC that day) (with a routine break at ~70ºC to test the cooling
system)
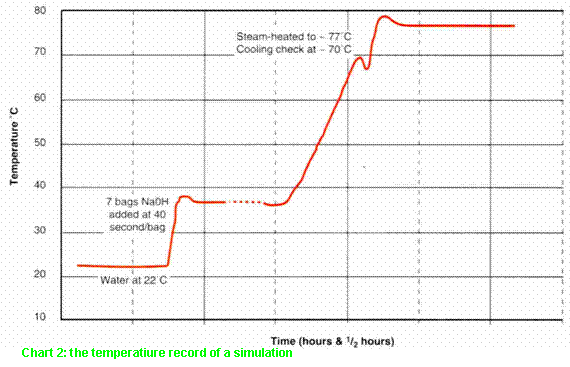
With hindsight, it
is instructive to compare Chart 1 with a simulation (for the subsequent
litigation) of these same conditions (Chart 2). Three abnormalities of Chart 1
(all absent from Chart 2) should have alerted the investigation team.
·
The
initial temperature rise took only 2½ minutes - under 25 seconds for each bag
(if all seven were emptied in this period), more than twice the normal charging
rate and even faster than the 3½ºC jump at 06:30 (c.f. 40 seconds/bag for the
simulation).
·
The rise
stopped at 34ºC and then meandered to 37ºC - without any of the features
normally associated with a controlled variable in an agitated vessel.
Furthermore, the cooling check at 70ºC did not produce a temperature drop (c.f.
Chart 2 - which did).
·
 If all 175kgs. dissolved in
2500 litres without cooling, the temperature should have reached 37ºC by 03:20am (q.v. Fig.1).
If all 175kgs. dissolved in
2500 litres without cooling, the temperature should have reached 37ºC by 03:20am (q.v. Fig.1).
These pre-accident abnormalities could
be explained if:
·
Less than
2500 litres water were present as the first bags of caustic soda were charged
at an abnormally fast rate.
·
The
vessel's agitator was not operating at that time.
·
About
40kgs. of caustic soda remained undissolved
and, later, formed an agglomerate at the bottom of the reactor.
This is a classic
scenario for the creation of layered solutions and the investigation team
should have realised the implication. They were equally blind to the shortcomings
of their preferred theory. Having calculated that a 2.7ºC rise
would result from adding 25kgs. to 2500 litres,
they ignored the corollary that the actual rise of 3½ºC could not be explained by the addition of
a single 25kg. bag.
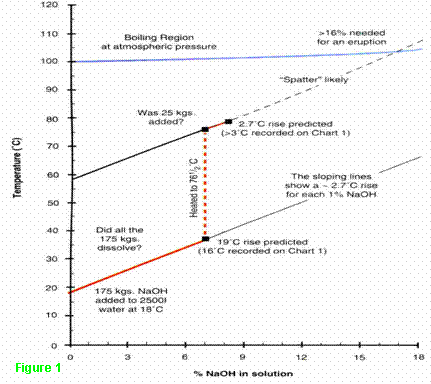 Fig. 1 (derived from vendors' data4 and
Chart 1) reveals another problem: that boiling would not occur if only one
extra bag were charged and that no less than nine bags would have to
be added to reach the 16% concentration required for a major eruption.
Fig. 1 (derived from vendors' data4 and
Chart 1) reveals another problem: that boiling would not occur if only one
extra bag were charged and that no less than nine bags would have to
be added to reach the 16% concentration required for a major eruption.
At the very most,
the company's hypothesis could have explained a large spatter of droplets as a recoil from the addition of solid caustic soda. It could
not account for a coherent splash or explain why, with the process worker in
the posture needed to empty a bag, this would have precisely targeted his head
and upper torso or had the force to penetrate the his
cotton overalls and gloves or to enter his goggles after hitting his forehead.
In contrast, the events are easily explained if the act
of opening the inspection cover disturbed, and triggered the mixing of, a
layered solution. An illustrative calculation was submitted in the High Court
to show that the process worker's recollections were consistent with this
scenario (the 'layering hypothesis').
After the incident, the temperature rose to ~80ºC and,
with 175kgs. of caustic soda dissolved in 2500 litres,
its concentration would have been 7% (represented on Fig. 2 by Point D). Before
this, there would have been two layers at 77ºC, at concentrations such that the
straight line joining the two points on Fig. 2 would pass through Point D. [The chart reading was
actually nearer to 76½ºC but, to avoid an unproductive cross-examination in the
High Court, 77ºC was used to illustrate the argument.]
75% is the maximum concentration of a caustic soda
solution at 77ºC so, if the agglomerate dissolved slowly at this temperature,
it could have become a dense 75% layer by 06:30am. For the purposes the High Court presentation, a lower
concentration was used (70% - represented by Point C2 on Fig. 2). With this
value for the heavier layer, the lighter layer (Point C1, where the extended
line from C2 to D cuts the 77ºC curve) would have had a concentration of 5.4%.
With 5.4% and 70% for the initial concentrations, the
volume ratio between the light and dense layers is ~40:1 (obtained by the
'lever rule'). In this instance, the heavier layer would occupy ~60 litres and
contain ~42kgs. of the initial 175kgs. charge of caustic soda.
It is noteworthy that 60 litres
is less than half the volume of the lower dished end of the reactor and that
the interface between the layers would have been only a quarter of the
cross-sectional area of the main body of the reactor. In the absence of
agitation, this would be an inherently stable situation - until the cover was
opened suddenly and a shock wave disturbed the 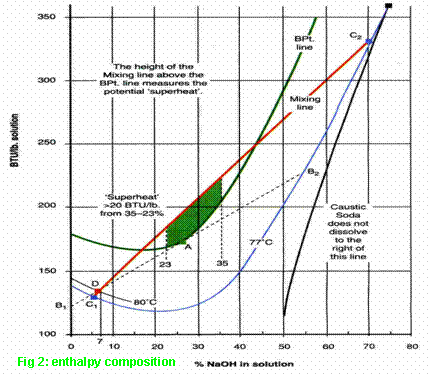 interface.
interface.
On Fig. 2, the line between C1 and C2 represents the
temperatures of all intermediate concentrations during mixing. This shows that
a disturbance at the interface would create transient concentrations in the
range from 42% to 18% (where boiling temperatures would be exceeded), that
vapour bubbles would form and, within fractions of seconds, the entire contents
of the reactor would be mixed.
Although the resultant energy release would have been
comparable with that of the Company's hypothesis, the consequences would have
been far more serious because the energy release would have been virtually
instantaneous and vaporisation would take place at the base of the reactor.
In theory (if vaporisation was truly instantaneous) the
volume of the eruption could exceed 5000 litres - twice the volume of the
reactor! In practice of course, the mixing and vaporisation would occur over
finite period and each bubble would be quickly quenched by its surrounding
liquid. The 'in theory' calculation is mentioned simply to indicate the potential volume that could be released
by an eruption resulting from caustic layering.
These calculations were presented to the High Court when
the case was heard in January 1998 (more than ten years after the incident).
There was no cross examination or challenge to this expert opinion since,
before the proceedings recommenced, the Company agreed to a 'substantial
settlement' - though, as customary on such occasions, without admitting any
liability.
John Cox
is a Consultant Chemical Engineer who has acted as an expert witness for
several process workers injured in accidents at chemical works.
REFERENCES
1 "Anhydrous
Caustic Soda", ICI Chlor-Chemicals, page 8
2 "Caustic
Soda Liquor", ICI Chlor-Chemicals, Fig. 9
3 Derived
from Reference 2 but only showing the curve for 77ºC.
4 Reference
1, page 13
 THE PHENOMENON of "Caustic
Layering", first identified in the 19th. Century, is rarely encountered
today. Suppliers of caustic soda have produced excellent technical literature
with clear warnings of the danger of creating weak and strong layers during
dissolution:
THE PHENOMENON of "Caustic
Layering", first identified in the 19th. Century, is rarely encountered
today. Suppliers of caustic soda have produced excellent technical literature
with clear warnings of the danger of creating weak and strong layers during
dissolution:



